WHO Approves First Malaria Vaccine
The World Health Organization endorsed the first malaria vaccine, presenting a way that could save the lives of children in Africa each year.
-
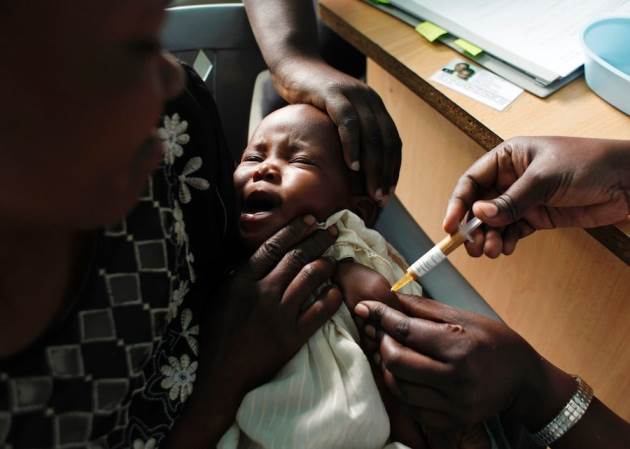
GlaxoSmithKline's new vaccine stimulates a child's immune system to combat Plasmodium falciparum
The World Health Organization approved the first malaria vaccine on Wednesday, launching a tool that might save the lives of tens of thousands of African children each year.
Malaria is one of the oldest and deadliest infectious diseases. It kills about 500,000 people per year, virtually all of whom are in Sub-Saharan Africa, including 260,000 children under the age of five.
GlaxoSmithKline vaccine
GlaxoSmithKline's new vaccine stimulates a child's immune system to combat Plasmodium falciparum, the deadliest of five malaria infections and the most common in Africa. The vaccine is not just the first for malaria; it is also the first for any parasite disease.
In clinical studies, the vaccine had a first-year efficacy of about 50% against severe malaria, but by the fourth year, it had decreased close to zero.
Furthermore, the trials did not assess the vaccine's effectiveness in reducing mortality, prompting some experts to question if it is a viable investment in countries with a plethora of other intractable issues.
However, severe malaria accounts for up to half of the malaria deaths and is regarded as a “reliable proximal indication of mortality,” according to Dr. Mary Hamel, who oversees the World Health Organization's malaria vaccine implementation program, adding that "I do expect we will see that impact."
2020 study findings
A modeling study revealed last year that if the vaccine were distributed to malaria-endemic nations, it might save 5.4 million infections and 23,000 deaths in children under the age of five per year.
A recent trial of the vaccine in combination with preventive medications given to children during high-transmission seasons revealed that the dual approach was far more efficient than either option alone in preventing severe disease, hospitalization, and death.
The availability of a malaria vaccine that is safe, reasonably effective, and ready for distribution is a "historic event," according to Dr. Pedro Alonso, director of the WHO's global malaria program.
Parasites are far more complex than viruses or bacteria, and the search for a malaria vaccine has been ongoing for over a century, he added. “It’s a huge jump from the science perspective to have a first-generation vaccine against a human parasite.”
Vaccine research
Vaccine candidates that never made it past clinical trials litter the landscape of malaria research. Bed nets, the most widely used preventive strategy, reduced malaria fatalities in children under the age of five by approximately 20% only.
Against this backdrop, some scientists believe that the new vaccine, despite its modest efficacy, is the best new advance in the fight against the illness in decades.
“Progress against malaria has really stalled over the last five or six years, particularly in some of the hardest-hit countries in the world,” said Ashley Birkett, head of the malaria programs at PATH, a nonprofit organization focused on global health.
With the new vaccine, “there’s potential for very, very significant impact there,” Dr. Birkett said.
Gavi, the global vaccination alliance, will then assess whether the vaccine is a worthy investment. If the organization's board of directors accepts the vaccine — which is unlikely given the vaccine's moderate efficacy and the organization's many competing priorities — Gavi will acquire the vaccine for nations who request it, a process that is expected to take at least a year.

 3 Min Read
3 Min Read








